Medical Devices
Distribution of Medical Devices
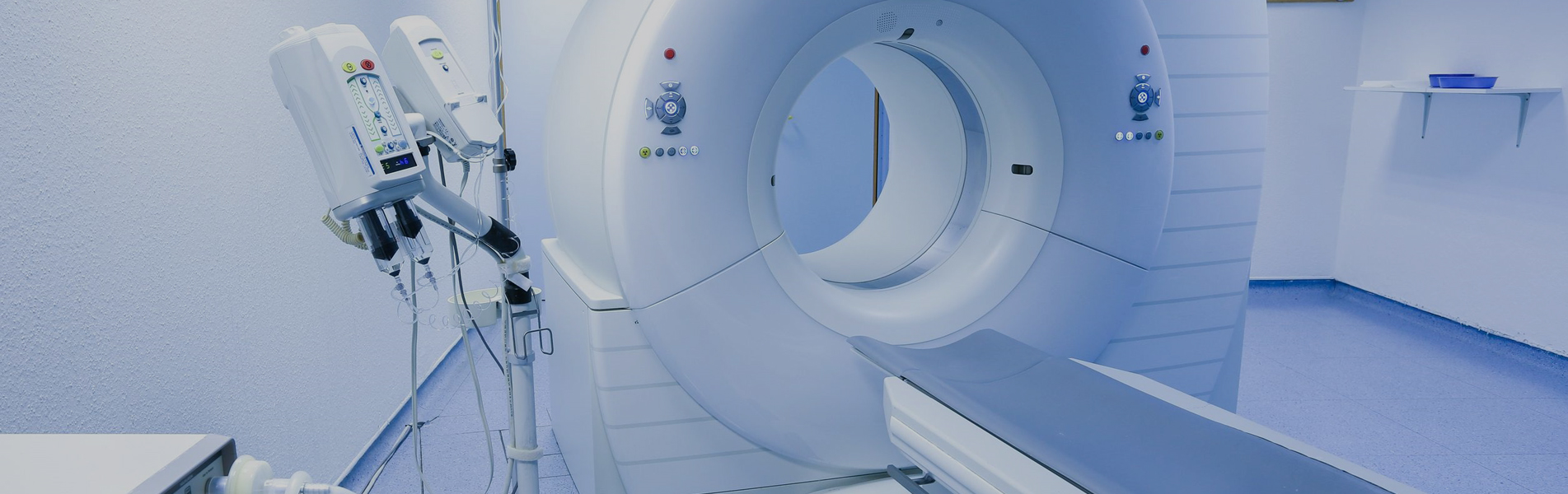
A medical device is an instrument, apparatus, equipment or even software intended, by its manufacturer, to be used in humans for purposes, in particular, of diagnosis, prevention, control, treatment of illness or injury.
A medical device for analyzing or examining in vitro samples from the human body (blood, urine, stool, etc.) is called an in vitro diagnostic medical device (IVDMD), such as a pregnancy test on a strip. or a medical analysis machine. A medical device designed to be implanted (in whole or in part) in the human body or placed in a natural orifice and to remain in place after the intervention is called an implantable medical device (IMD), such as for example a total hip prosthesis . A medical device which depends for its proper functioning on a source of electrical energy or any source of energy other than that which is generated directly by the human body or gravity, is called active medical device (AMD), as by example an ultrasound scanner. If it is implantable, we will speak of an active implantable medical device (AIMD), such as for example a pacemaker.
According to the World Health Organization (WHO), medical devices are essential for safe, effective prevention, diagnosis, treatment of disease and rehabilitation. The achievement of health-related development goals, including the Sustainable Development Goals for development, involves the manufacture, regulation, planning, evaluation, acquisition, management and use of medical devices that are of high quality, safe and adapted to their context of use.
WHO is therefore committed to facilitate access to and use of quality, safe and appropriate medical devices in the spirit of primary healthcare. In line with these objectives, EQUIMED GROUP SARL offers to its customers a wide range of products.