PharmaVigi-WestAf
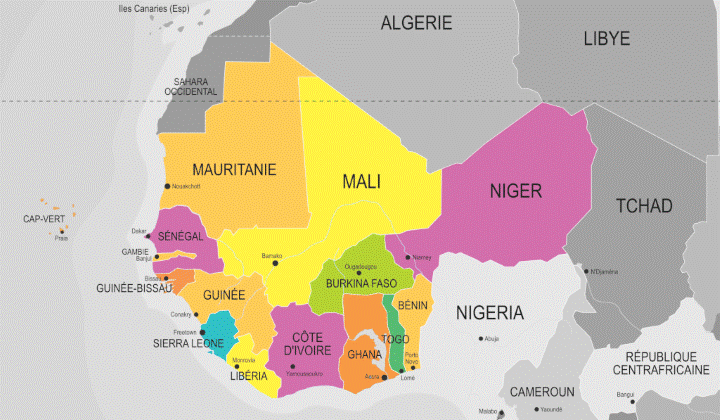
West Africa or Western Africa is the westernmost region of Africa.
The United Nations defines Western Africa as the 16 countries of Benin, Burkina Faso, Cape Verde, Cote d’Ivoire, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Senegal, Sierra Leone and Togo, as well as the United Kingdom Overseas Territory of Saint Helena, Ascension and Tristan da Cunha.
The population of West Africa is estimated at about 381 million people in 2018. [United Nations]
This makes West Africa a huge market for the pharmaceutical industry and also an import source of data for the WHO Programme for International Drug Monitoring (PIDM).
Togo
Pharmacovigilance reporting systems is the core data-generating system of pharmacovigilance, relying on healthcare professionals and patients to identify and report any suspected adverse effects from medicines to their local or national pharmacovigilance centre or to the manufacturer. Togo is a full member of the WHO Programme for International Drug Monitoring (PIDM). “Direction de La Pharmacie, du Médicament et des Laboratoires” (DPML) is the Togo Drugs Regulatory Authority. The national pharmacovigilance centre, Centre National De Pharmacovigilance Togo (CNPV TG), is located in DPML.
Pharmacovigilance
DPML
Quartier Administratif – Avenue du 2 Février
Angle Rue Aniko Palako, Face Direction Générale de la Police Nationale (D.G .P.N.)
01 B.P. 1661 Lomé, Togo
Tél.: +228 23 20 91 52
Fax: +228 22 22 07 99
CNPV TG
E-mail: cnpvtg@gmail.com
Facebook Page: @cnpvtg
For good reporting, the CNPV TG has set up a standard form available from its focal points and also made available to caregivers. To this end, the CNPV TG periodically organizes training workshops to promote spontaneous notification. Download ADRs reporting form here…
Clinical Trials
CBRS
Tel: +22 8 22 21 38 01
Fax: +228 22 22 07 99
BP 386 Lomé Togo
Before a medicine is authorised for use, evidence of its safety and efficacy is limited to the results from clinical trials, where patients are selected carefully and followed up very closely under controlled conditions. This means that at the time of a medicine’s authorization, it has been tested in a relatively small number of selected patients for a limited length of time. Clinical Trials are regulated in Togo by a Bioethics Committee named “Comité de Bioéthique pour la Recherche en Santé” (CBRS) also located in DPML.
Other WestAf Countries
West Africa as a region is a major actor in terms of global pharmacovigilance activities. Benin, Burkina Faso, Cape Verde, Cote d’Ivoire, Ghana, Guinea, Liberia, Mali, Niger, Nigeria, Senegal, Sierra Leone and Togo are full members of the WHO Programme for International Drug Monitoring (PIDM) and Gambia, Guinea-Bissau are associated members. Mauritania is not taking part at the present time but may join the group in the future.
Data from West Africa are part of VigiBase and countries like Burkina Faso, Ghana and Cote d’Ivoire are enrolled in the Recognise Adverse Drug Reactions on the Web (WEB-RADR) grant-supported project and using the web-application Med Safety.
On this page EQUIMED GROUP is sharing with professionals the contact details of the regulatory authorities involved in pharmacovigilance in the rest of West African countries.
Bénin
Agence Béninoise de Régulation Pharmaceutique
AKPAKPA, DONATIN, Cotonou, Bénin
(229) 21 33 08 82
contact@abrp.bj
www.abrp.bj
Burkina Faso
Direction Générale de la Pharmacie, du Médicament et des Laboratoires (DGPML)
03 BP: 7009 Ouagadougou 03 Burkina Faso
Tel: 00226 50 32 46 60 / +226 70 20 35 15
Fax: 00 226 50 31 44 76
Web: www.dgpml.sante.gov.bf
Cabo Verde
Direction de la Pharmacie, Ministère de la Santé
BP 47 PRAIA
Tel: (+238) 61 01 12 Fax : (+238) 61 39 91
edith@santos.ms.gov.cv
Cote d’Ivoire
Autorité Ivoirienne de Régulation Pharmaceutique
Cocody Rieviera Bonoumin ,Rue I 89, Quartier Avocatier
secretariat@dpml.ci
TEL +225 22 53 28 73 / +225 22 49 71 09
Boite Postal : 08 BP 3535 ABIDJAN 08
www.dpml.ci
Gambia
Medicine Control Agency
54 Kairaba Avenue, Opposite American Embassy, The Gambia
info@mca.gm
www.mca.gm
Ghana
Food and Drugs Authority
No. 17 Indian Ocean Street, Nelson Mandela Avenue, Accra
(+233) – 302-233200/ 235100
(+233) – 0299802932/3 (Hotline)
0800151000 (Toll free)
info@fda.gov.gh
https://www.fdaghana.gov.gh/
Guinée
Ministère de la Santé Publique, DNPL
BP 1490, CONAKRY
Tel: (+224) 628 45 20 28 / +224 628 53 28 80
Fax: (+224) 628 45 20 50
drsouare_kabine@yahoo.fr
Guinée Bissau
Direction des Services Pharmaceutiques
Ministère de la Santé Publique, BP 50, BISSAO
Tel: (+245) 20 11 07
Fax: (+245) 20 12 85
Liberia
Liberian Medicines and Health Products Authority
LMHRA, PO Box 1994 VP Road, Old Road, Sinkor Monrovia, Liberia
Tel: (+231) (0) 886562019
info@lmhra.org
lmhra2010@gmail.com
Notification: https://www.lmhra.org/submit-safety-report
https://www.lmhra.org/
Mali
Ministère de la Santé, Direction de la Pharmacie et du Médicament
Rue 569 (limite Darsalam centre commercial) Commune III Bamako, BPE 5202
Tel: (+223) 66 73 76 35 / (+223) 20 22 65 70
Fax: (+223) 20 23 24 63
dpm@dirpharma.org
couliyaya2003@yahoo.fr
www.dirpharma.org
Mauritanie
Direction de la Pharmacie et du Médicament, Ministère de la Santé Publique et des Affaires Sociales (MSAS)
BP 2763, NOUAKCHOTT
Tel: (+222) 525 23 12
Fax: (+222) 525 11 97 (+222) 529 66 79
directionpharmacie@yahoo.fr
Niger
Ministère de la Santé Publique, Direction de la pharmacie
BP 623, Niamey-Niger
Tel: (+227) 20 72 47 00
info@dphmt-msp.ne
Notification: https://www.dphmt-msp.ne/declaration-alerte
https://www.dphmt-msp.ne/
Sénégal
Direction de la Pharmacie et du Médicament
153, rue Mousse Diop X Victor Hugo
BP 6150, DAKAR
Tel: (+221) 33 822 44 70
Fax: (+221) 33 869 42 49
info@dirpharm.net
www.dirpharm.net
Sierra Leon
Pharmacy Board of Sierra Leone
Central Medical Stores Compound
New England Ville, Freetown
P.M.B. 322, Sierra Leon
(+232 22) 228497, 225983, 229346
(+232 22) 224526
info@pharmacyboard.gov.sl
www.pharmacyboard.gov.sl

